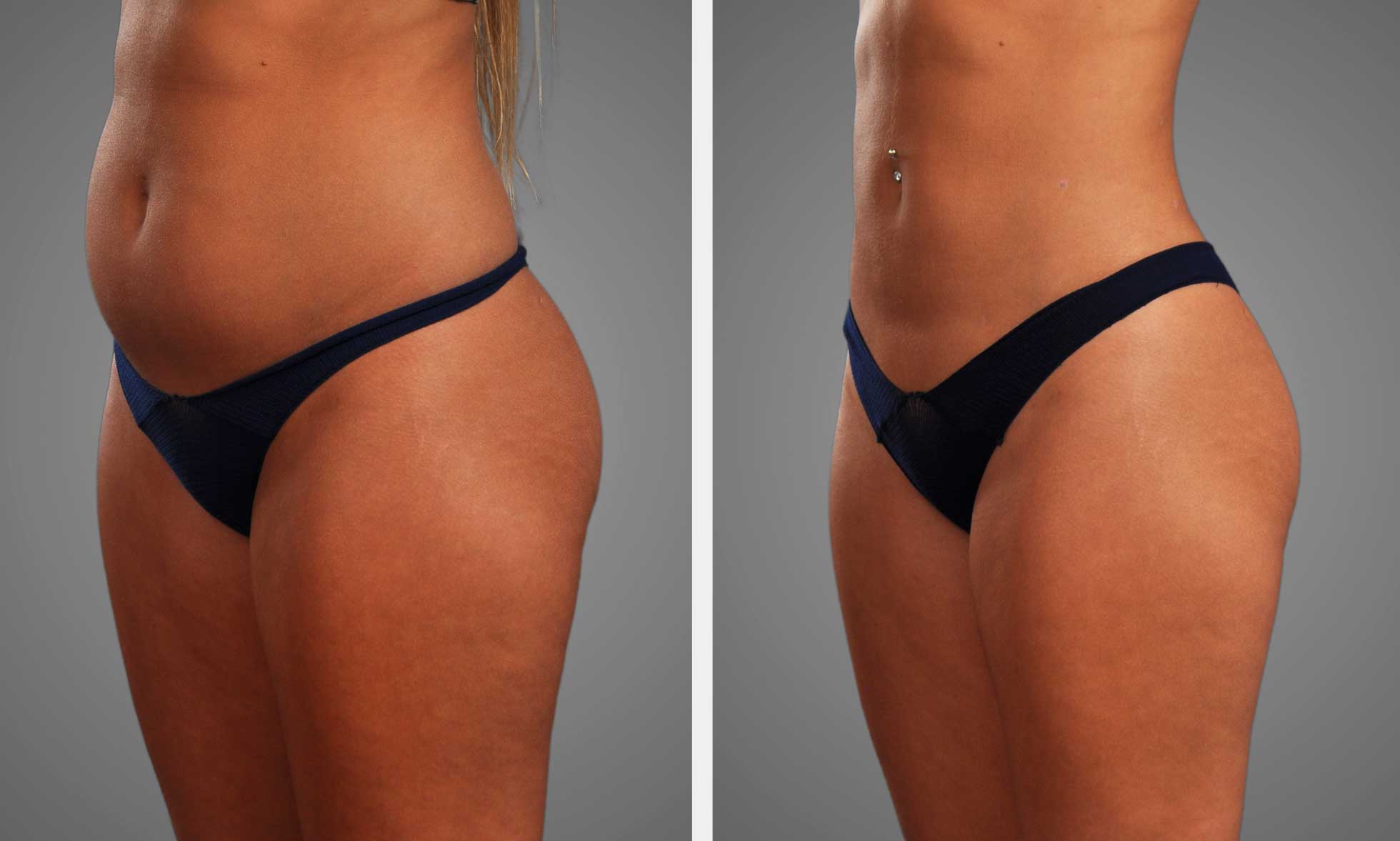
Key Takeaways
- Hormonal imbalances, especially involving estrogen and progesterone, drive abnormal subcutaneous fat accumulation in lipedema and make affected adipocytes unusually sensitive to sex hormones.
- Estrogen receptor alterations and elevated local estrogen synthesis support adipogenesis, inflammation, and fibrosis. Tracking estrogen signaling can inform specific treatments.
- Progesterone resistance can diminish these anti-inflammatory and fluid-regulating influences, exacerbate swelling and tissue inflammation, and make progesterone status relevant to symptom management.
- Other metabolic factors include insulin resistance and thyroid dysfunction, both of which disrupt lipid metabolism and can intensify local estrogen activity, so test insulin sensitivity and thyroid function regularly.
- Now, life stages like puberty, pregnancy, perimenopause, and menopause frequently coincide with symptom onset or exacerbation. Charting your own hormonal milestones helps customize treatment and track fluctuations.
- Smart management marries hormone-targeted meds and lifestyle interventions to optimize metabolic health and supportive therapies, with routine hormone and metabolic marker monitoring to customize care.
Hormonal changes and their role in lipedema details how fluctuations in estrogen, progesterone, and other hormones impact fat cells and lymphatic function.
Lipedema typically initiates or exacerbates during puberty, pregnancy, or menopause when hormonal levels shift.
Studies tie hormonal signals to abnormal fat growth, inflammation, and water retention in the legs and arms.
The next sections summarize existing evidence, diagnostic indicators, and practical strategies for symptom management.
The Hormonal Connection
Hormonal shifts influence how lipedema fat develops, expands, and defies traditional weight-loss efforts. Below are the key hormone systems involved and how they alter fat behavior in lipedema versus normal fat regulation.
1. Estrogen’s Influence
Estrogen receptor dysregulation in lipedematous adipocytes stimulates increased adipogenesis and increased lipid storage. Adipose tissue has ERα and ERβ, and research demonstrates that both receptors influence leptin and adipocyte development. In lipedema, the balance tips, generating more fat cell formation and chronic low-grade inflammation.
A shift toward ERβ dominance seems associated with fibrosis and cell dysfunction. Local estrogen production in fat, through steroidogenic enzymes, maintains tissue estrogen levels after the post-menopause systemic drop. That local production supports signaling loops that encourage lipogenesis and decelerate lipolysis.
Estrogen modifies lipid metabolism by up-regulating genes for lipid uptake and storage and by shifting the differentiation of precursor cells to adipocytes, so subcutaneous depots enlarge disproportionally. Single-cell RNA sequencing identifies adipocyte subtypes in lipedema that may be differentially responsive to estrogen, explaining tissue heterogeneity and why fat accumulates despite calorie restriction.
2. Progesterone’s Role
Typically, progesterone modulates inflammation, aids in fluid homeostasis, and promotes healthy adipose tissue function. In lipedema, there is evidence of progesterone resistance: adipocytes show reduced response to progesterone signals. When progesterone action drops, tissue swelling and inflammation persist, and extracellular matrix alterations ensue.
That makes fat pads matted and painful. Progesterone’s effects contrast with estrogen’s: where estrogen tends to promote adipocyte growth and fibrosis in vulnerable tissue, progesterone would normally limit those processes. Loss of progesterone control therefore shifts the balance toward continued fat accumulation and exacerbated symptoms.
3. Insulin Resistance
Insulin resistance frequently turns up in lipedema and exacerbates anomalous fat storage. Compromised insulin signaling tampers with effective lipolysis, so fat cells expand and retain triglycerides. Insulin resistance connects to increased local estrogen production via disturbed steroid metabolism, providing a positive feedback loop that drives hyperplasia and inflammation.
There’s an important hormonal connection too. Measuring insulin sensitivity, fasting glucose, and related markers provides management data and can direct metabolic therapies in addition to local treatments.
4. Thyroid Function
Thyroid hormones influence basal metabolic rate, lipid oxidation, and adipocyte differentiation. Hypothyroid states suppress metabolic rate and divert lipid management toward storage, which can exacerbate lipedema. Thyroid dysfunction can affect fat cell health and remodeling post adipocyte apoptosis and regeneration.
Regular thyroid screening helps paint a complete picture of hormonal factors and facilitates holistic treatment planning.
Life’s Hormonal Shifts
Hormonal milestones throughout life tend to coincide with shifts in lipedema symptoms and disease trajectory. Puberty, pregnancy, perimenopause, and menopause introduce fluctuations in estrogen, progesterone, and other endocrine signaling that can trigger the onset or exacerbate existing lipedema. Research and clinical reports document that approximately 67% of women experience significant symptom exacerbation around menopause, while approximately 20% are first diagnosed during that life phase.
Thyroid dysfunction is common, impacting approximately 30% of lipedema patients, so hormone checks should encompass thyroid testing.
Puberty is a common period for the onset of symptoms. Estrogens rising encourage fat cell proliferation and regional fat deposition. In predisposed people, this can translate into early development of painful, symmetric fat deposits on the hips, thighs, and arms. Symptoms typically include uneven limbs, fragile bones, and pain.
Pregnancy causes long-term shifts in estrogen and progesterone as well as fluid and weight variation. Some patients note obvious symptom exacerbation, enhanced leg heaviness, and hypersensitivity. These are accompanied by wide hormonal swings during perimenopause, which can destabilize adipose tissue regulation.
Menopause typically results in lower estrogen baseline but greater volatility in both local fat function and inflammation, which many experience as lipedema sign advancement.
Estrogen and progesterone influence adipocyte quantity, dimensions, and metabolic activity. Estrogen controls fat distribution and local blood flow. Changes in it can alter adipose tissue’s inflammatory tone and lymphatic load.
When estrogen fluctuates, fat cells could accumulate more lipid, call in macrophages, and change the extracellular matrix, which could exacerbate lipedema nodularity and pain. Hormonal shifts regulate immune signaling. Shifts in cytokines and inflammatory mediators during this time can make you more prone to tenderness and swelling.
Thyroid hormone shifts alter metabolism and fluid handling and can imitate or worsen lipedema. Include TSH, free T4, and potentially relevant antibodies in evaluation.
Common symptom changes during major hormonal events include:
- More pain and tenderness in affected areas occur during puberty, pregnancy, perimenopause, and menopause.
- Rapid growth of affected limb circumference or new fat deposits.
- More frequent or severe bruising and capillary fragility.
- More fluid retention and heaviness occur particularly with pregnancy and perimenopause.
- Less reactive to diet and exercise, with excessive loss of fat in other locations.
- New or worsening thyroid-related fatigue and weight issues.
Develop your own life’s hormonal map – connecting symptom changes with key hormonal milestones – to inform care decisions. Follow events, labs (estrogen, progesterone, TSH), symptom onset, and treatment response. Share that timeline with clinicians to customize management that considers hormonal status, as treatment impact can change with life stages.
Beyond Reproductive Hormones
Lipedema is commonly positioned as being driven primarily by estrogen and progesterone. However, other hormonal systems are central to the pathophysiology of disease onset and progression. Adrenal hormones, androgens, and metabolic hormones like leptin and cortisol interact with local estrogen signaling in fat tissue, influencing cell growth, inflammation, and fibrosis.
Knowing these connections sheds light on why symptoms wax and wane with stress, weight fluctuations, and life phases such as menopause. Adrenal hormones and androgens affect adipose behavior directly and through modification of estrogen pathways. Cortisol, secreted by the adrenal glands when you’re stressed, encourages fat storage, decreases protein synthesis, and increases blood sugar.
Chronic cortisol excess can exacerbate adipocyte hypertrophy and inflammation in lipedema, and it can redistribute fat to the limbs. Rogens, though at low levels in most affected women, are converted locally by aromatase to estrogens, including local estradiol. Both cortisol and adrenal androgens modify receptor expression and enzyme activity in fat, such as by increasing aromatase or by shifting the local steroid balance through changes to 17β-HSD enzymes.
Metabolic hormones such as leptin and insulin connect fat mass to inflammation and metabolic regulation. Leptin rises with expanding adipose tissue and can be a driver of low-grade inflammation. It causes insulin resistance, which inflammatory cytokines and estrogen receptor shifts both encourage, and which cuts muscle glucose use and increases fat retention.
Mitochondrial dysfunction associated with estrogen deficiency decreases basal metabolic rate, which can promote sarcopenia and insulin resistance. Both of these further fuel fat accumulation in lipedematous tissue. Like reproductive hormones, non-reproductive hormones do not act in isolation but cross-talk with estrogen and progesterone signals.
Lipedema demonstrates an ER imbalance in adipose. ERα decreases and ERβ increases in states of estrogen deficiency, in which inflammation and fibrosis are more likely. Local estradiol production by aromatase and 17 β-HSD1 is upregulated, and inactivation by 17 β-HSD2 is downregulated in the presence of progesterone resistance.
With progesterone no longer able to modulate adipose tissue, intracrine estradiol keeps inflammatory pathways active. This local hyperestrogenism echoes the mechanisms in endometriosis, adenomyosis, and fibroids, which are conditions marked by tissue-level estrogen production that sustains disease.
This explains why systemic hormonal imbalances amplify local processes. Menopause-related estrogen decline shifts receptor balances and mitochondrial efficiency, modifying metabolism and tissue repair. Chronic stress increases cortisol, which encourages adipocyte expansion and inflammatory signaling.
All these systemic changes in concert dictate severity and pace of progression and why some patients deteriorate after life events such as menopause, major stress, or weight gain. A holistic approach to hormonal health matters for management. Testing should consider adrenal, androgenic, and metabolic hormones along with sex steroids.
Treatments that address stress, improve insulin sensitivity, and limit local estrogen synthesis may slow progression and reduce symptoms.
The Endocrine System Web
The endocrine system is a web of glands and tissues that secrete hormones to regulate metabolism, inflammation, and fat storage. This web-like behavior helps explain why lipedema exhibits such complex hormonal associations when one node is disrupted. Hormonal shifts alter fat cell behavior, immune cell recruitment, and extracellular matrix remodeling.
For instance, dysregulated estrogen signaling in adipose tissue can boost lipid uptake and locally support inflammation, whereas thyroid dysfunction reduces basal metabolic rate and can potentially exacerbate fat storage in vulnerable individuals. These interactions make a single imbalance rarely act in isolation. Estrogens, progestogens, thyroid hormones, insulin, cortisol, and local steroid production all interplay and shape the lipedema phenotype.
| Hormone | Effect on adipose tissue | Relevance to lipedema |
|---|---|---|
| Estrogen (estradiol) | Promotes adipocyte differentiation, modulates lipolysis, affects inflammation via ERα/ERβ | Estrogen receptors in adipose tissue link estrogen signaling to fat metabolism and inflammation in lipedema |
| Progesterone | Regulates estrogen action, anti-inflammatory in some contexts | Progesterone resistance seen in lipedema can raise estradiol activity and favor inflammation |
| Thyroid hormones (T3/T4) | Increase basal metabolic rate, support lipid mobilization | ~30% prevalence of thyroid dysfunction in women with lipedema; lower thyroid output can worsen fat retention |
| Insulin | Stimulates lipid storage, inhibits lipolysis | Hyperinsulinemia promotes fat accumulation and may worsen regional adiposity patterns | | Cortisol | Alters fat distribution, can increase visceral fat and inflammation | Chronic stress hormones may modify extracellular matrix and inflammation in adipose tissue | | Local steroidogenesis | Local conversion of precursors to active sex steroids in adipose | Extra-gonadal steroidogenesis may modulate local estrogen/progesterone balance. The role in lipedema is unclear |
Hormonal homeostasis maintains equilibrium in fat deposition by aligning hunger, energy expenditure, and tissue-level activation. Loss of that balance, whether it be through menopause, endocrine disease, or local receptor changes, can tip the scale in favor of the abnormal, painful fat deposits found in lipedema.
Progesterone resistance, for instance, eliminates a check on estradiol effects and primes the tissue toward a pro-inflammatory phenotype with more macrophage infiltration and fibrosis. That inflammatory drive then further changes local steroid signaling, creating a self-reinforcing loop.
Clinical assessment should look beyond a single lab value. A useful workup includes a thyroid panel, sex hormones with attention to free fractions, fasting insulin or HOMA-IR, cortisol rhythm when indicated, and markers of inflammation. Imaging and tissue biopsy can add context where fibrosis or macrophage load is suspected.
Shared mechanisms with estrogen-dependent gynecological diseases suggest coordinated care with gynecology when symptoms overlap. Diet and metabolism count as well. Early evidence suggests that keto or low-carb strategies decrease inflammation and relieve symptoms in some individuals. Additional trials are required before strong recommendations can be made.
Hormonal Management Strategies
Hormonal approaches aim to correct the shifts in sex steroids and related pathways that drive fat deposition, inflammation, and lymphatic changes seen in lipedema. Early estrogen support, careful progesterone use, thyroid tuning, and attention to extra-gonadal steroid production can each change the disease course and symptom burden.
Estrogen modulation and replacement. It is critical to begin estrogen replacement early, during the menopausal transition or within a few years of menopause, in order to avoid disrupting the estrogen receptor homeostasis. Estrogens regulate energy balance and glucose homeostasis, so ensuring physiologic estrogen levels can decrease the tendency to shift fat storage in targeted areas.
Estrogen replacement may additionally lower local inflammation, decelerate fibrosis, and aid in preserving lymphatic function in individuals with lipedema. Routes and doses should be selected to approximate physiologic levels and to minimize systemic risks. Transdermal delivery is frequently preferred for stable blood levels and decreased thrombogenic risk relative to some oral preparations.
Progesterone support and selectors. Progesterone typically dampens adipose responses to estrogens. When this control breaks down, intracrine estradiol can maintain inflammation in adipose tissue. Progesterone can assist in bringing you back into balance, but type is key.
Drospirenone exerts an anti-adipogenic effect by blocking mineralocorticoid receptors, preventing preadipocyte differentiation and triglyceride accumulation. This positions drospirenone-containing regimens as a possible option wherever hormonal contraception or replacement is appropriate, always balanced against personal cardiovascular and metabolic risks.
Thyroid optimization. Thyroid hormones sculpt basal metabolism and lipid management. Mild thyroid insufficiency can exacerbate fat deposition and fluid retention. Evaluating and treating subclinical deficiencies can reduce symptoms and metabolic markers.
Seek symptom-guided normalization of free T4 and T3 and adjust levothyroxine or combination therapy accordingly. Tackling extra-gonadal steroidogenesis. Peripheral tissues can produce steroids locally, which may perpetuate adipose inflammation and increase.
Strategies that reduce local steroid production or action via systemic hormone modulation, receptor antagonists, or enzyme inhibitors may be required when peripheral steroidogenesis is believed to fuel progression.
- Diet and metabolic shifts: Adopt a low-carbohydrate or ketogenic-style plan to promote fat use for energy and improve insulin sensitivity. Electrolytes and kidney function should be monitored at initiation.
- Weight and body composition: Combine resistance training with moderate aerobic work to preserve lean mass and improve glucose handling.
- Sleep and stress: Prioritize regular sleep and stress reduction to lower cortisol pulses that favor fat deposition.
- Alcohol and medications: Limit alcohol and review drugs that promote weight gain or fluid retention.
- Micronutrients and supplements: Ensure adequate vitamin D, magnesium, and omega-3 intake to support metabolic and inflammatory control.
- Lymphatic care and manual therapies maintain lymph flow through compression, MLD, and skin care to reduce fibrosis and inflammation.
Monitor baseline and serial hormone panels and metabolic markers, including estrogen, progesterone, SHBG, free T4/T3, TSH, fasting glucose, insulin, HbA1c, and lipid profile. Leverage trends in these data to optimize personalization of therapy and timing.
Mix medical, surgical, and lifestyle care for optimal fat management and symptom control.
Future Research Directions
A concise summary of unknowns directs future empirical research on hormonal change and lipedema. Major goals are to map local hormone action in affected fat, to test how therapies alter tissue function, to identify genetic drivers of hormone sensitivity, and to establish consensus clinical and research measures of hormonal and metabolic status.
Call for more studies on estrogen receptor expression, local estrogen metabolism, and steroidogenic enzyme activity in lipedema adipose tissue.
Direct measures of ERα, ERβ, and GPER in lipedema versus healthy adipose are needed. Tissue-level assays should combine protein localization with mRNA and functional measures, such as receptor activity following estradiol exposure.
Quantify local steroidogenesis, such as aromatase, 17β-HSD, and sulfatases, in subcutaneous fat and lymphatic tissue. Cross-sample life stages, such as pre-, peri-, and postmenopause, to observe how extra-gonadal steroid production shifts during endocrine transitions.
Use paired serum and tissue biopsies to connect local metabolism with circulating hormones. Animal models and ex vivo cultured adipocytes can probe whether blocking or enhancing enzyme activity alters adipocyte size, fibrosis, or immune cell infiltration.
Propose investigating the impact of hormone therapy and advanced lipedema treatments on adipocyte function and disease progression.
Test typical hormone therapies — combined estrogen-progestin, estrogen-only, and anti-androgens — for their impact on fat cell differentiation, lipid storage, and inflammation in lipedema. Conduct randomized or controlled observational studies that follow limb volume, pain, and histology before and after therapy.
Explore advanced interventions such as liposuction, lymphatic therapy, and receptor-specific anti-inflammatory drugs for effects on steroidogenic enzyme expression and receptor profiles. Integrate clinical endpoints with metabolic tests like insulin sensitivity and lipid panels to tie treatment effects to whole-body metabolism.
Encourage research into the genetic and molecular mechanisms underlying hormonal sensitivity and adipose tissue dysfunction in lipedema.
In future research directions, large-scale sequencing and genome-wide association studies should search for variants associated with hormone signaling, extracellular matrix remodeling, or lymphatic function. Single-cell RNA sequencing of affected tissue may uncover cell types that alter receptor expression or inflammatory signaling.
Functional studies need to test candidate genes in cell culture and small animal models to demonstrate cause and effect.
Suggest developing standardized protocols for assessing hormonal status and metabolic health in lipedema research and clinical practice.
Create consensus panels to recommend which hormones, inflammatory markers, and metabolic tests to run, and standardize biopsy methods and imaging for adipose assessment. Include diet and microbiome sampling and test diets like low-carbohydrate, low-fat, modified Mediterranean ketogenic diets and record effects.
Incorporate patient-reported outcomes to link biology with quality of life and guide best practice.
Conclusion
Hormonal shifts have an obvious connection to lipedema. Estrogen and its related signaling influence fat cells, blood vessels, and tissue fluid. Puberty, pregnancy, and menopause tie to flare-ups and accelerated fat accumulation. Other glands and hormones provide additional layers of risk and alter symptom presentation. Current treatments combine lifestyle changes, compression, and targeted care to relieve pain and slow advancement. Studies now target clearer indicators, improved medications, and personalized regimens.
Give weight to simple steps that help now: steady protein-rich meals, low-impact exercise like walking or water class, fit compression, and regular check-ins with a clinician who knows lipedema. Check with your provider for such studies or trials that fit your profile.
Frequently Asked Questions
What role do hormones play in lipedema?
Hormones affect fat distribution, fluid retention, and inflammation. Estrogen and other pathways are frequently involved, suggesting that hormonal changes may play a role in lipedema development and progression.
Which hormonal changes commonly trigger lipedema symptoms?
Puberty, pregnancy, and menopause are typical triggers. These life stages alter estrogen and progesterone levels, frequently exacerbating the pain, swelling, and fat deposits in affected regions.
Are reproductive hormones the only ones involved in lipedema?
Reproductive hormones are key, but these other systems, thyroid, cortisol, insulin, and adipokines, can influence fat metabolism, inflammation, and tissue fluid, which all help explain lipedema features.
Can hormone testing help diagnose or manage lipedema?
Hormone testing can find imbalances that exacerbate symptoms. Diagnosis is still clinical. Tests guide targeted treatments with specialist review.
Do hormone treatments improve lipedema?
Hormone therapies are another thing that can reduce symptoms for certain individuals, as they may help correct imbalances. Outcomes differ. Treatment should be individualized and overseen by an endocrinologist or specialist familiar with lipedema.
What non-hormonal strategies complement hormonal management?
Compression therapy, manual lymphatic drainage, exercise, nutrition, and weight-stable approaches all reduce symptoms and improve function. These complement hormone care and can frequently provide symptomatic relief.
Where is research on hormones and lipedema headed?
She is researching estrogen signaling, fat cell biology, lymphatic effects and genetic links. The hope is that future studies will help to improve targeted medical therapies and clearer diagnostic markers.